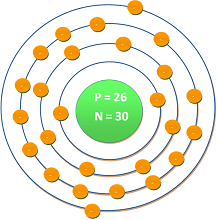
| Atomic number | : | 26 |
| Atomic mass | : | 55.85 g.mol-1 |
| Electronegativity according to Pauling | : | 1.8 |
| Density | : | 7.8 g.cm-3 at 20 °C |
| Melting point | : | 1536 °C |
| Boiling point | : | 2861 °C |
| Vanderwaals radius | : | 0.126 nm |
| Ionic radius | : | 0.076 (+2) nm ; 0.064 (+3) nm |
| Isotopes | : | 8 |
| Electronic shell | : | [Ar] 3d6 4s2 |
| Energy of first ionisation | : | 761 kJ.mol-1 |
| Energy of second ionisation | : | 1556.5 kJ.mol-1 |
| Energy of third ionisation | : | 2951 kJ.mol-1 |
| Standard Potential | : | - 0.44 V (Fe2+ / Fe) ; 0.77 V (Fe3+ / Fe2+) |
| Discovered by | : | The ancients |
Periodic Table of Elements