The process of electrolysis is used in depositing a layer of one metal over other. The process of depositing a layer of one metal over the surface of another metal by passing electric current in called electroplating.
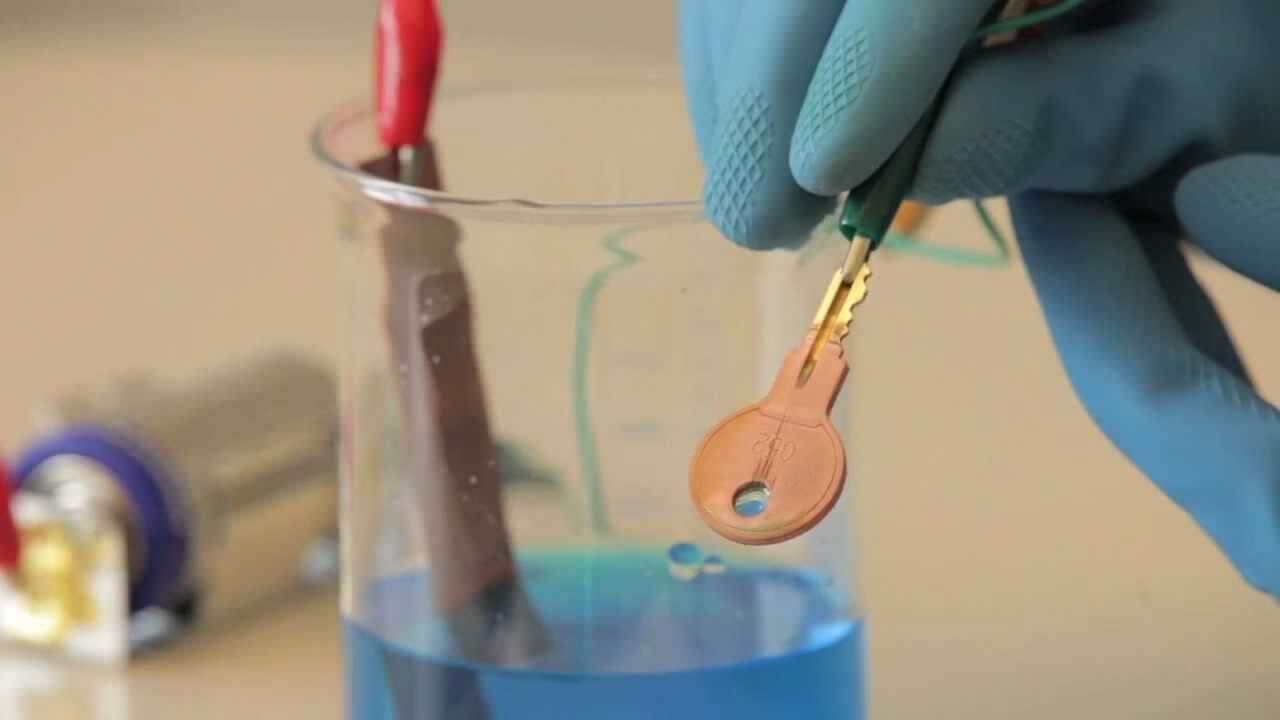
Electroplating of metals is done to protect the metals from corrosion and for decoration purpose. For example, the vessels of iron are electroplated with copper or silver or gold to make it attractive and to protect from rusting.
Electroplating of an Iron Object with Copper Metal
To deposit a layer of copper metal on an iron object, take a glass jar and fill it with copper sulphate solution (electrolyte). Connect the plate of copper metal to the positive terminal (anode) of battery and iron object to the negative terminal (cathode) of the battery. Now, dip these two electrodes in the solution of copper sulphate. When electric current is passed through the CuSO4 solution, you will find that a thin layer of copper metal is deposited on the cathode (iron object) and an equivalent amount of copper is lost by the anode (copper plate) and is dissolved into the solution.

Test Your Understanding and Answer These Questions:
- What is electroplating process?
- Explain the process of electroplating of an iron object with copper metal?
