The metal obtained by the methods in previous article is usually impure. So, it is to be purified. The method used for refining of metal depends on the nature of metal and impurities present in it. Some common methods which are used for purification of impure metals are:
1. Distillation Method
This method is useful for purification of those volatile metals which have low boiling points such as zinc and mercury. In this method, the impure metal is heated to its boiling point in a vessel. The vapours of metal thus formed are collected and cooled in a separate vessel to get pure metal. The impurities being non-volatile remain behind.
2. Liquation Method
By this method, those metals can be purified which have low melting point. In this method, the block of Impure metal is placed on the top side of sloping floor of a furnace and heated gently. Due to high temperature, the fusible metal melts and flows down to the bottom of the sloping floor while the non-fusible impurities remain behind on the floor. Finally, the pure metal is collected from the bottom of the sloping floor.
3. Electrolytic Refining
Many metals like Cu, Zn, Pb, Cr, Ni, Ag, and Au are refined electrolytically for refining of an impure metal by electrolysis.
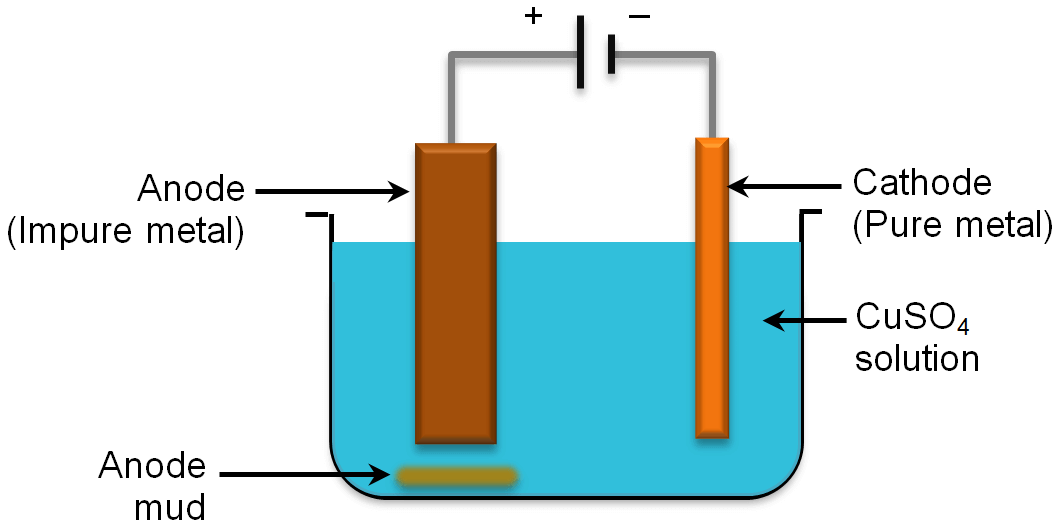
We shall understand electrolytic refining of metals by taking the example of refining of copper. In case of copper, a thick block of impure copper is made anode and a thin block of pure metal is made cathode and copper sulphate solution is used as an electrolyte. On passing electric current, pure copper metal from the electrolyte solution deposits on the cathode. At the same time, an equal amount of impure copper dissolves from anode into the electrolyte solution. The soluble impurities settle down in the solution below the anode and are called as anode mud.
Test your understanding and answer these questions:
- Explain electrolytic refining of copper metal.
