There are two common methods which are used to get metals of very high purity. These are:
1. Zone Refining
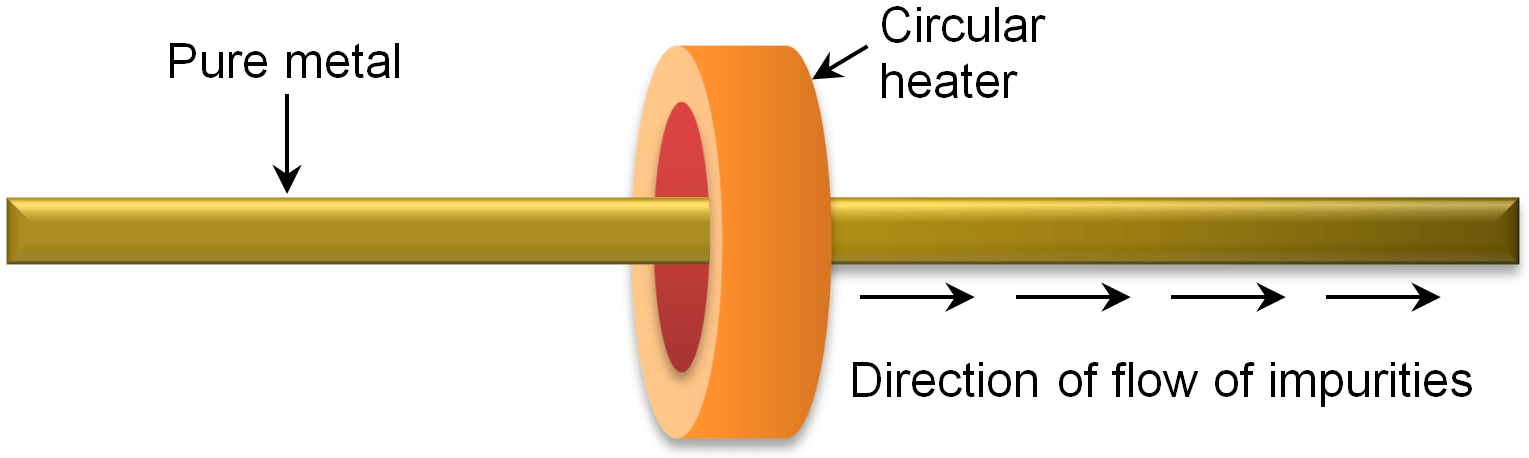
By this method, those metals are purified which require high purity such as germanium, silicon and gallium. In this method, impure metal is taken in the form of a rod. A circular heater is fitted around it. The function of the heater is to molt the impurities present in the germanium rod. For this purpose, the heater is slowly moved along the length of the rod to the right side. As the heater moves to the right side on the metal rod, the molten impurities also move to the right side of the rod. Ultimately the impurities reach the extreme right end of metal rod and collect there. This end of rod containing impurities is finally cut off and discarded.
2. Van Arkel Method
This method is used for refining of impure titanium metal. In this method, the impure titanium metal is heated to a temperature of 250°C with iodine. At this high temperature, titanium reacts with iodine to form a volatile compound titanium tetraiodide. Impurities do not react with iodine so these are left behind.
Ti + 2I2 ![]() TiI4
TiI4
(Titanium + Iodine → Titanium tetraiodide)
The vapours of titanium tetraiodide are then passed over a hot filament of tungsten having a temperature of about 1400°C. At this high temperature, titanium tetraiodide decomposes to form pure titanium metal and iodine is set free.
TiI4 ![]() Ti + 2I2
Ti + 2I2
(Titanium tetraiodide → Titanium + Iodine)
In this way, pure titanium metal is formed which deposits over the tungsten filament and can be removed easily.
Test your understanding and answer these questions:
- What is zone refining process?
- What is van arkel method?
