There are more than 115 elements known at present and these can be divided into two main groups:
Metals
Metals are the elements which can form positive ions by losing electrons. For example, Na is a metal because it can form positive ion (Na+) ion by losing an electron from its outermost shell. In the same way Iron, aluminium, copper, gold, silver, magnesium, potassium and calcium are also metals because all of these elements can form positive ions by losing electrons.
It should be noted that the oxides of metals are basic in nature. All metals are solid at room temperature except mercury which is liquid metal.
Non-metals
Non-metals are the elements which can form negative ions by gaining electrons. For example, oxygen is a non-metal because it can form negative ion (O2-) ion by gaining two electrons. In the same way Hydrogen, Carbon, Sulphur, Silicon and Phosphorus are also non-metals because all these elements can form negative ions by gaining electrons.
Unlike metals the oxides of non-metals are acidic or neutral in nature. There are 22 non-metals. At room temperature non-metals exist in solid, liquid as well as in gaseous form. Out of 22 non-metals 10 non-metals are solid 8 (e.g. carbon, Sulphur and phosphorous), 11 non-metals are gases (e.g. hydrogen, nitrogen, oxygen and chlorine), whereas only one non-metal (bromine) is a liquid non-metals.
Position of Metals and Non-metals in the Periodic Table
In the periodic table metals are placed on the left hand side and centre whereas non-metals are placed on right side of periodic Table.
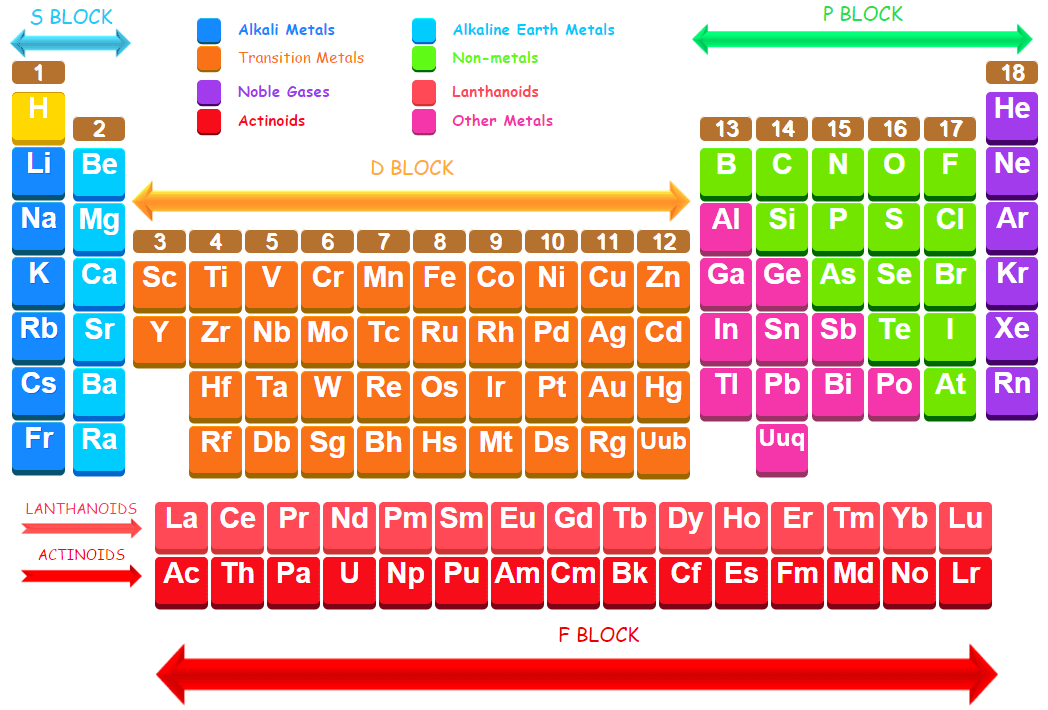
The metals and non-metals are separated by the elements which show the properties of both metals and non-metals and are called metalloids.
Test your understanding and answer these questions:
- What are metals and nonmetals?
