Hydrogen is the lightest element. An atom of hydrogen contains one electron and one proton. It is a unique element because it can lose as well as gain one electron. When an atom of hydrogen loses one electron it acts as metal. On the other hand, when an atom of hydrogen gains one electron, it acts as non-metals. A molecule of hydrogen gas consists of two atoms of hydrogen (H2). It is the most abundant element of atmosphere. On earth, hydrogen is present in the form of water, natural gas and oil. All the Organic compounds contain hydrogen along with carbon, oxygen and nitrogen. Even Sun is made up of hydrogen. Hydrogen is found in the form of three isotopes. These are:
1. Protium (1H1)
2. Deuterium and (2H1)
3. Tritium (3H1)
Laboratory Preparation of Hydrogen Gas
In the Laboratory hydrogen gas is usually prepared by the reaction of zinc granules with dilute hydrochloric acid.
Chemical Equation:
Zn(s) + 2HCl(aq) → ZnCl2 (aq) + H2(g)
(Zinc + Hydrogen chloride → Zinc chloride + Hydrogen gas)
Procedure
To prepare hydrogen gas in the laboratory take a conical flask and add some zinc granules in it. Now, pour dilute hydrochloric acid in conical flask with the help of a thistle funnel.
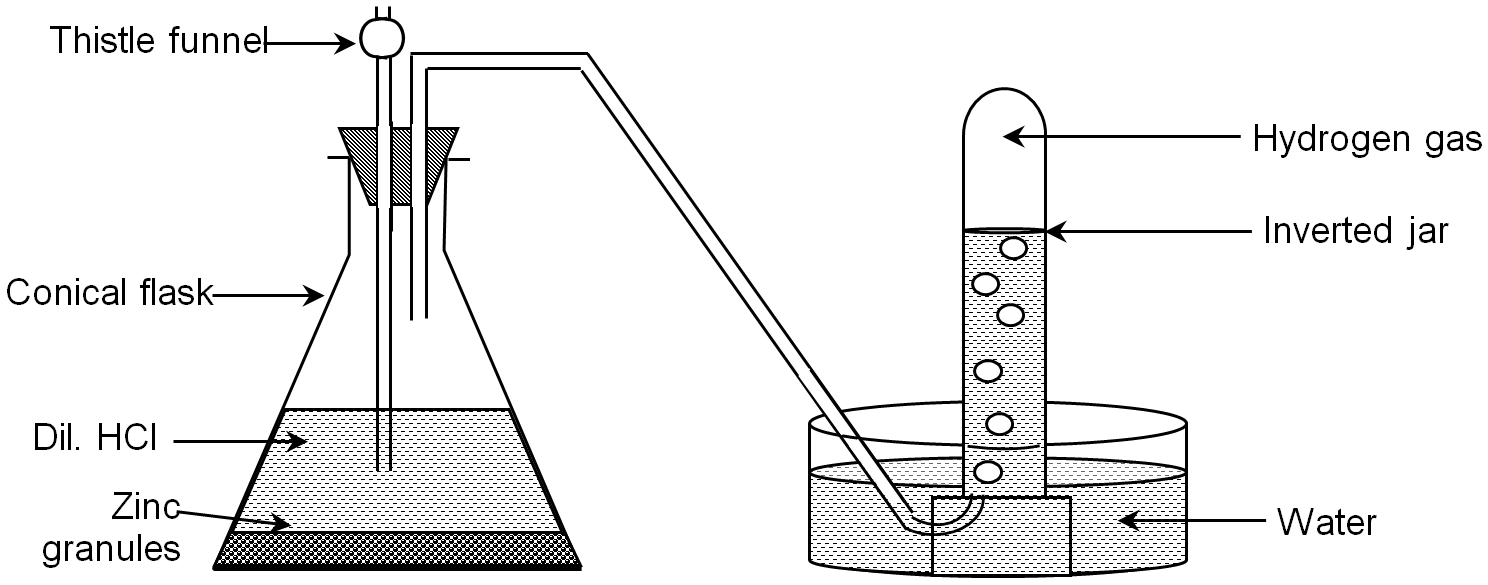
Granules of zinc react with dilute hydrochloric acid to produce hydrogen gas which is collected in an inverted gas jar by downward displacement method.
Preparation of Hydrogen Gas on Industrial Scale
Hydrogen gas is prepared on industrial scale by a process called steam reforming. In this method, methane gas is mixed with steam and passed over nickel catalyst at 800°C temperature and 30 atmosphere pressure to produce carbon monoxide and hydrogen.
CH4 + H2O → CO + 3H2
(Methane + Water → Carbon monoxide + Hydrogen)
Carbon monoxide is then reacted with more steam to produce more hydrogen gas.
CO + H2O → H2 + CO2
(Carbon monoxide + Water → Hydrogen + Carbon dioxide)
Physical Properties of Hydrogen Gas
1. It is a colourless, odourless and tasteless gas.
2. It is non-poisonous and insoluble in water.
3. It is lighter than air and is the lightest of all gases.
Chemical Properties of Hydrogen
Hydrogen is a neutral gas i.e. it is neither acidic nor basic in nature.
1. Combustion: Hydrogen is highly combustible gas i.e. it catches fire very easily.
2H2(g) + O2(g) → 2H2O(g)
(Hydrogen + Oxygen → Water)
2. Reaction with Chlorine: Hydrogen gas reacts with chlorine in the presence of sunlight to form hydrogen chloride.
H2 + Cl2 → 2HCl
(Hydrogen + Chlorine → Hydrogen chloride)
3. Reaction with Nitrogen: Hydrogen reacts with nitrogen gas in the presence of catalyst iron to form ammonia gas. This process of preparation of hydrogen is called Haber’s process. It should be noted that this reaction is a reversible reaction.
N2 + 3H2 → 2NH3
(Nitrogen + Hydrogen → Ammonia)
4. Reaction with Metal Oxide: Hydrogen gas is a good reducing agent. So, it reacts with many metal oxides to reduce them into metals. For example, when hydrogen gas is passed over heated copper oxide it reduces copper oxide to copper metal
CuO(s) + H2(g) → Cu(s) + H2O(g)
(Copper oxide + Hydrogen → Copper metal + Water)
Uses of Hydrogen
- It is used in welding metal.
- Liquid hydrogen is used as fuel in rockets.
- Hydrogen is used in the manufacture of ammonia by Haber’s process.
- Hydrogen is used in the manufacture of vegetable ghee by hydrogenation of vegetable oils.
- It is used in the manufacture of methanol.
Test your understanding and answer these questions:
- How hydrogen gas is prepared in laboratory?
