Allotropy may be defined as the existence of an element in two or more different forms. These different forms of an element are called allotropes. Sulphur also exhibits allotropy and exists in the form of two allotropes:
1. Rhombic sulphur
2. Monoclinic sulphur.
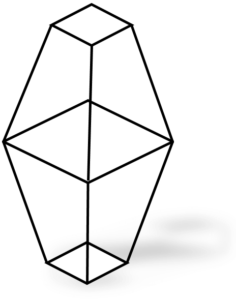
Rhombic Sulphur

Monoclinic Sulphur
These two allotropes of sulphur have same chemical properties but different physical properties. The allotropes have different physical properties because they have different crystal shapes. The shape of crystals of rhombic sulphur is octahedral shape, whereas monoclinic sulphur has needle shaped crystals.
The difference in shapes of two allotropes of sulphur is due to the difference in the packing arrangement of S8 molecules. In rhombic sulphur the S8 molecules fit tightly into each other, whereas in monoclinic sulphur, the S8 molecules are piled up on top of each other.
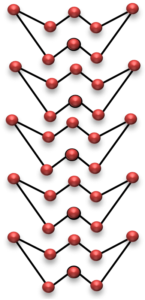
Rhombic Sulphur
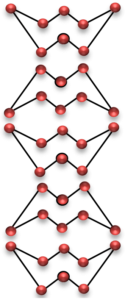
Monoclinic sulphur
It should be noted that rhombic sulphur is stable below 96°C whereas monoclinic sulphur is stable above 96°C.
Test your understanding and answer these questions:
- Define allotropy.
- Give two allotropes of sulphur.
- Explain allotropy in sulphur.
- What do you understand by rhombic and monoclinic sulphur?
