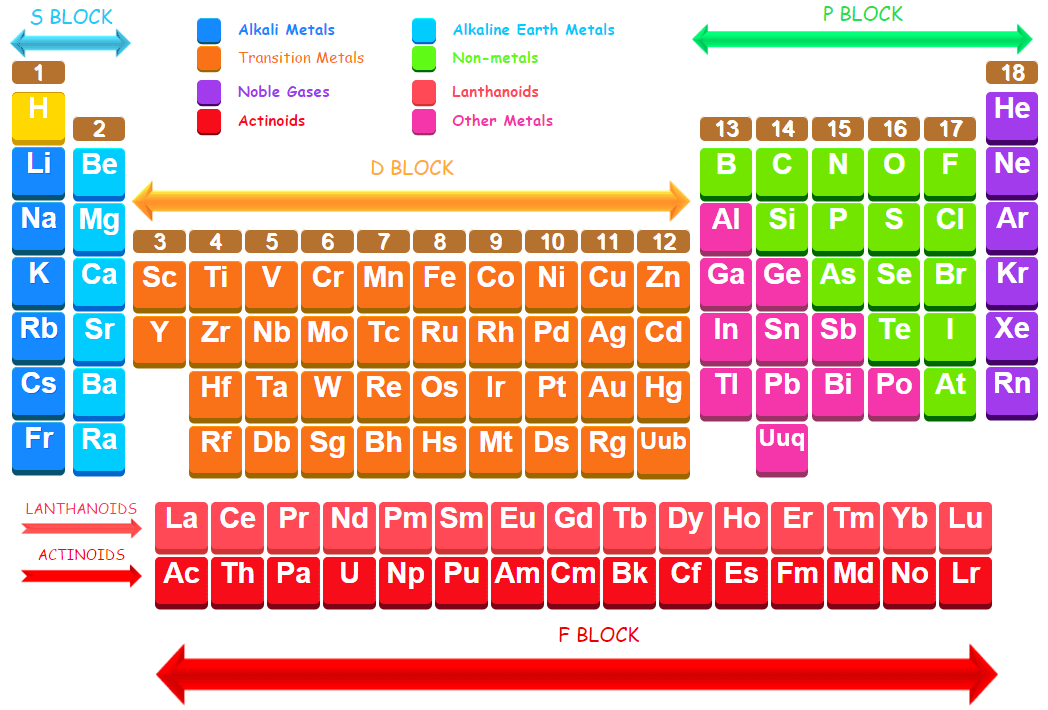
Modern Periodic Law of element may be defined as the Physical and chemical properties of the elements are periodic functions of their atomic numbers. It means, when the elements are arranged in the order of their increasing atomic numbers, it is observed that the elements of similar properties recur at regular intervals or periodically. As a result of this, the elements fall in certain groups and lead to an arrangement called the modern periodic table of elements. It must be noted that elements are arranged in the periodic table in order of atomic numbers because the atomic number is the most fundamental property of an element. The systematic arrangement of elements in the modern periodic table according to their atomic number helps in justification of isotopes of elements at one place.
Test your understanding and answer these questions:
- What is modern periodic law?
