The elements in which the last electron enters the p-orbital of their outermost energy level are called p block elements. The elements of groups 13 to 18 involving addition of one (ns2 np1), two (ns2 np2), three (ns2 np3), four (ns2 np4), five (ns2 np5), and six (ns2 np6) electrons respectively in p-orbitals are called p block elements. s-orbitals are already filled in their atoms. The general electronic configuration for the atoms of p block elements may be written as : ns2 np1-6
The elements of s-and p-block are collectively called representative elements. The elements of last group (18) having ns2 np6 configuration are called noble gases. All the orbitals in the valence shell of the noble gases are completely filled and they have no tendency to lose or gain electrons. Therefore, the noble gases exhibit very low reactivity. Preceding the noble gas family are two chemically important groups of non-metals. These are halogens (group 17) and chalcogens (group 16). These two groups of elements can readily accept one and two electrons respectively to attain noble gas configurations and form univalent and divalent negative ions.
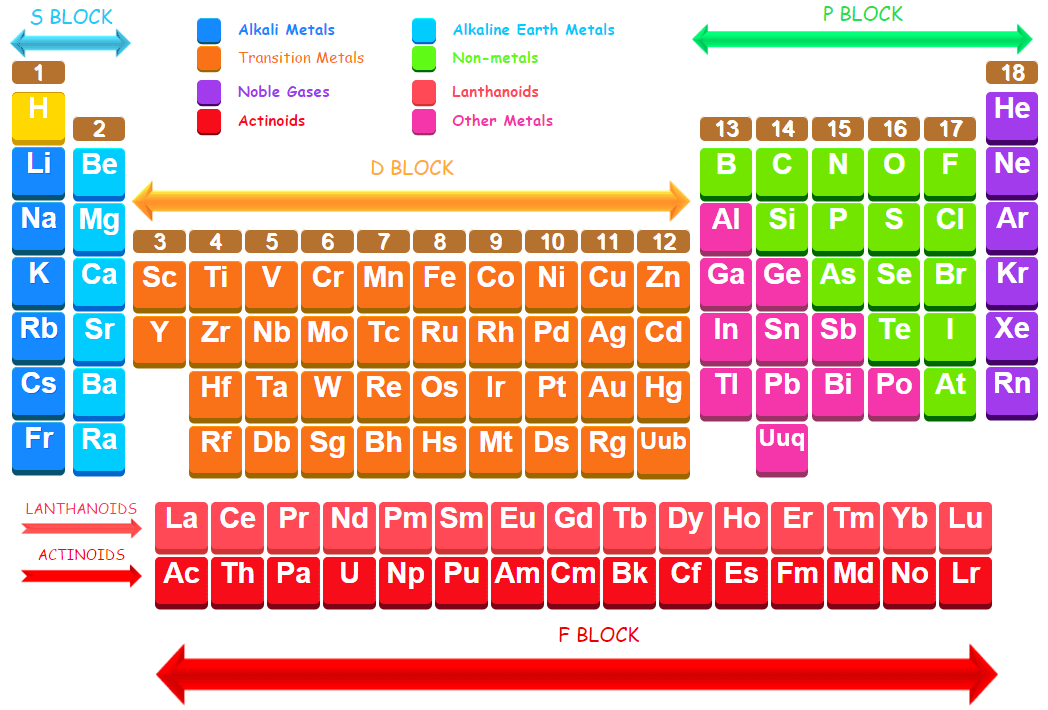
General characteristics of p-block elements
- They include both metals and non-metals
- Their ionization enthalpies are relatively high as compared to s-block elements.
- They form mostly covalent compounds.
- Some of them show more than one oxidation states in their compounds.
Test your understanding and answer these questions:
- What are noble gases?
- What is the general electronic configuration of p block elements?
