The elements in which the last electron enters the d-orbitals of their last but one (called penultimate energy level) constitute d-block elements. This block consists of the elements lying between s and p blocks starting from fourth period and onwards. They constitute groups 3 to 12 in the periodic table. In these elements the outermost shell contains one or two electrons in their s-orbital (ns) but the last electron enters the last but one d-subshell i.e., (n-1)d. The general electronic configuration for the atoms of d-block elements may be written as (n-1) d1-10 ns0-2
These elements are also called transition elements because transition elements form a bridge between the chemically active metals of s-block elements and non-metals elements of p block. Therefore, they represent transition (change) in behaviour and take their familiar name “transition elements”.
The d-block comprises three complete rows of ten elements and one incomplete row. These rows are called first, second and third transition series which involve the filling of 3d, 4d and 5d –orbitals respectively. These series are also called transition series.
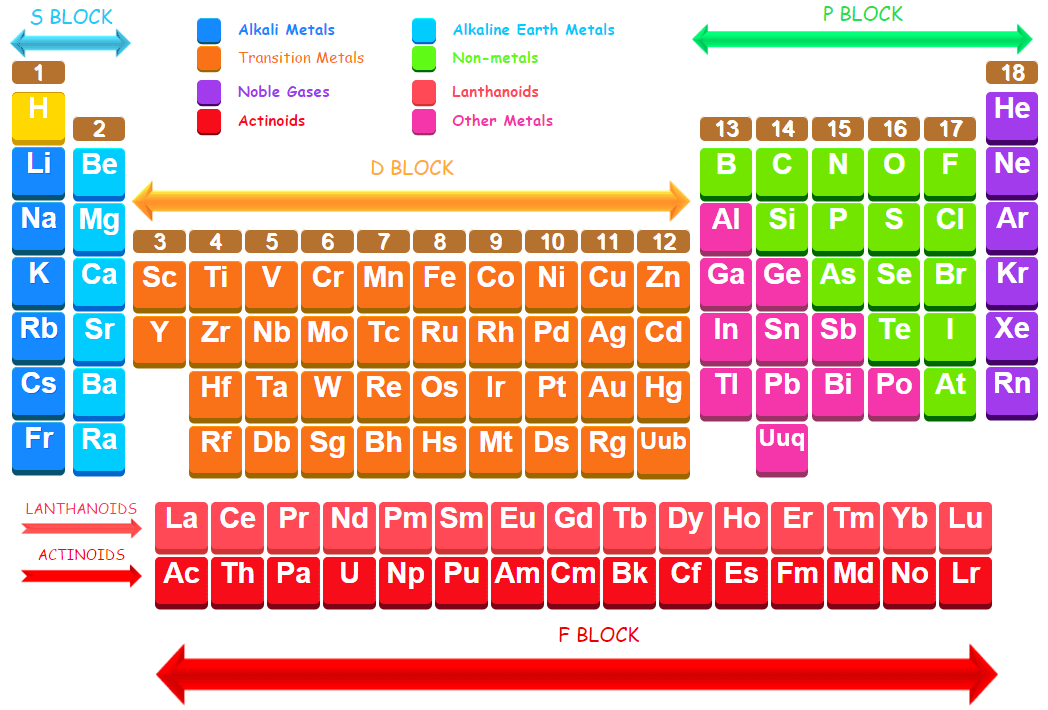
Elements of First transition series
Scandium (Z = 21) to Zinc (Z = 30)
(4th period : 3d- orbitals are gradually filled)
Elements of Second transition series
Yttrium (Z = 39) to Cadmium (Z = 48)
(5th period : 4d-orbitals are gradually filled)
Elements of Third transition series
Lanthanum (Z = 57), Hafnium (Z = 72) to Mercury (Z = 80)
(6th period : 5d- orbitals are gradually filled)
Elements of Fourth transition series
Actinium (Z = 89), Rutherfordium (Z = 104) to Copernicium (Z = 112)
(7th period : 6d- orbitals are gradually filled)
General characteristics of d-block elements
- They are metals having high melting and boiling points.
- Most of them form coloured compounds.
- They have a good tendency to form complex compounds.
- Their compounds are generally paramagnetic.
- They exhibit several oxidation states.
- Most of the transition elements such as Mn, Ni, Co, Cr, V, Pt and their compounds are used as catalysts.
Test your understanding and answer these questions:
- What are transuranium elements?
- Why are d block elements called transition elements?
- What is the general electronic configuration of d block elements?
