In addition to classifying the elements into s block, p block, d block and f block, the elements can be classified in three categories on the basis of their properties as: (1) Metals (2) Non-metals and (3) Metalloids.
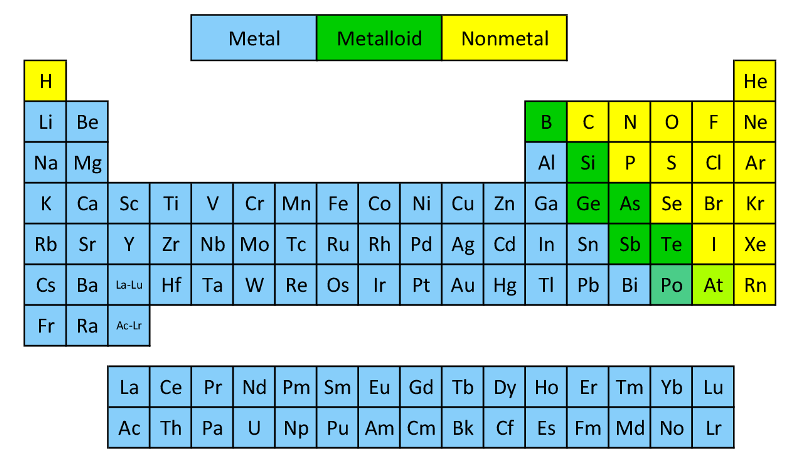
Position of Metals on the Periodic Table
Metals appear on the left hand side of the Periodic Table, They have high melting and boiling points and are generally solid at room temperature (mercury is an exception which is a liquid at room temperature. They are good conductors of heat and electricity. They are malleable (can be flattened into thin sheets) and ductile (can be drawn into wires). They have bright luster.
Position of Nonmetals on the Periodic Table
Nonmetals are present on the right hand side of the periodic table. They are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions). The only liquid non-metal is bromine. Most non-metals are brittle and are neither malleable nor ductile.
Metalloids or Semimetals
The elements silicon, germanium, arsenic, antimony, tellurium, etc. show properties characteristic of both metals and non-metals. These are called metalloids or semimetals. These are present diagonally on the p block of modern periodic table.
Trends in metallic and non-metallic character
In general, the metallic increases as we go down a group and non-metallic character increases as we move from left to right across a period in the Periodic Table.
Test your understanding and answer these questions:
- Name a liquid metal.
- Name a liquid nonmetal.
- What are metalloids?
- What is the position of metals on periodic table?
- What is the position of nonmetals on periodic table?
- What is the position of metaloids on periodic table?
