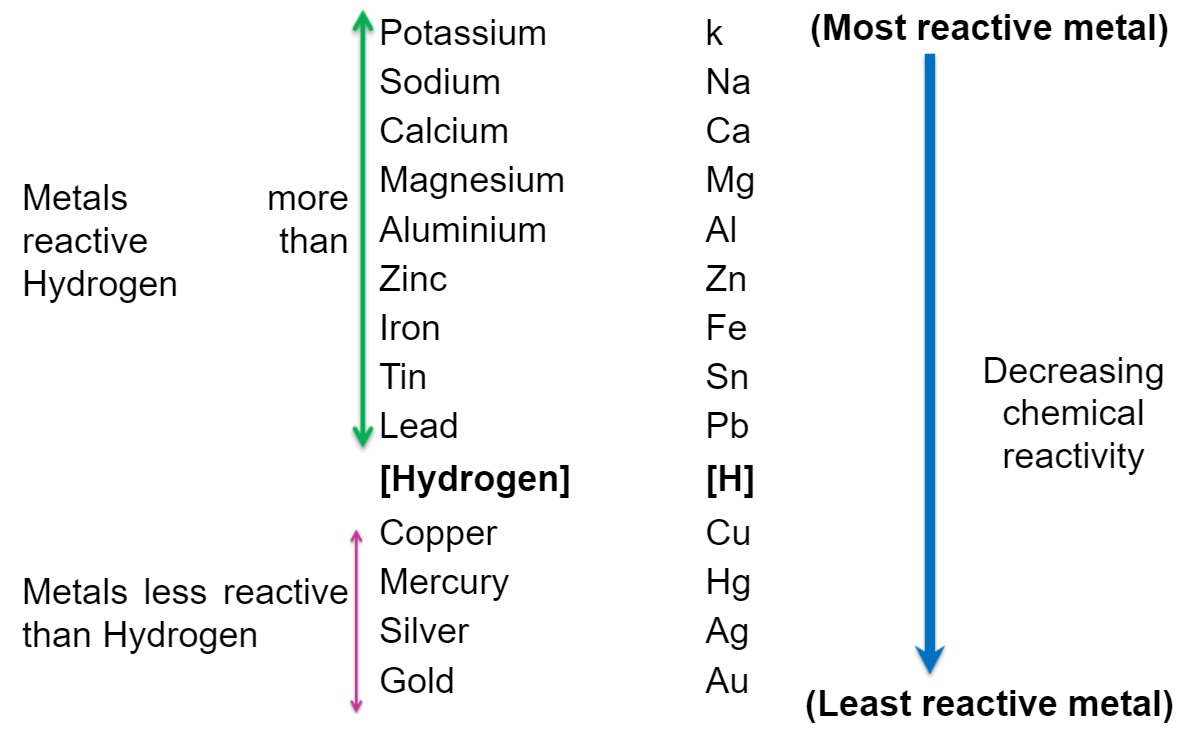
All metals are not equally reactive. Some metals are more reactive than others. The arrangement of metals in the order of their decreasing reactivities is called the reactivity series or activity series of metals. See table.
As potassium metal is present at the top of reactivity series so it is the most reactive metal. While, gold is present at the bottom of the reactivity series so gold is the least reactive metal. It should be noted that all the metals which are present above hydrogen are more reactive metals and hence found in combined state in earth. And the metals which are present below hydrogen are less reactive and hence found in free state in earth.
As the metals present above hydrogen in the activity series are more reactive than hydrogen so these can displace hydrogen from water or acids and produce hydrogen gas. On the other hand, metals present below hydrogen in the activity series are less reactive than hydrogen so these can not displace hydrogen from water or acids to produce hydrogen gas.
Table of Reactivity series or activity series of metals
Why some metal are more reactive than others?
Reactivity of a metal depends upon the ease with which its atom can lose the valence electrons present in its outermost shell to other substances. If a metal atom can lose its valence electron easily it will react rapidly with other substances and hence it will be a reactive metal. On the other hand, if a metal atom cannot lose its valence electrons easily it will react slowly with other substances and hence will be less reactive.
Test your understanding and answer these questions:
- What is activity series of metals?
