Aluminium is the most abundant metal in the earth’s crust. It is a reactive metal so it occurs in combined state as its oxides and fluorides. The important ores of aluminium are:
1. Bauxite Al2O3.2H2O (aluminium oxide dihydrate)
2. Cryolite Na3AlF6 (sodium aluminium fluoride)
The most important ore of aluminium is bauxite. In India bauxite is found in Mumbai, Jammu & Kashmir, Jabalpur, Kolhapur, Mirzapur and Ranchi.
Extraction of Aluminium Metal from Bauxite Ore
1. Purification of Bauxite Ore
Bauxite contains impurities such as sand and iron oxide. These impurities are removed from bauxite by Baeyer’s process. The pure bauxite obtained in Baeyer’s process is called alumina.
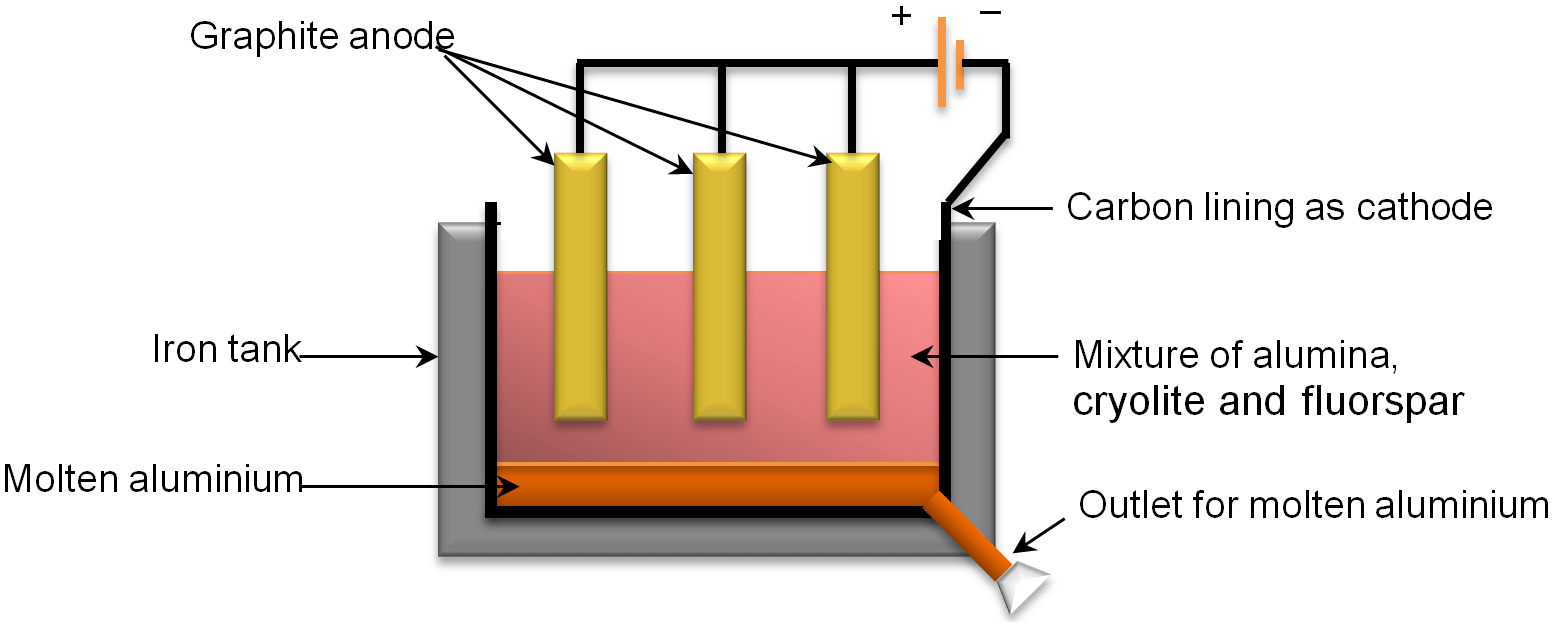
2. Electrolysis of Aluminium Oxide
Aluminium metal is extracted by the electrolytic reduction of molten alumina (aluminium oxide) in an iron tank. The melting point of alumina is very high. So, to reduce the melting point of alumina cryolite and fluorspar are added in it.
The mixture of alumina, cryolite and fluorspar is then put into a specially made electrolytic cell. The electrolytic cell is made up of iron which is lined with a layer of carbon from inside. This layer of carbon acts as cathode. A large number of carbon rods are also dipped in the mixture of alumina, cryolite and fluorspar. These rods act as an anode. When an electric current is passed through electrolytic cell, the mixture melts and molten aluminium metal is produced at the cathode and oxygen is liberated at the anode. Finally, the molten aluminium metal collects at the bottom of the iron tank from where it is collected and cooled to make it solid.
Al2O3 → 2Al+3 + 3O-2
(Aluminium oxide → Aluminium ion + Oxide ion)
At Cathode:-
Al+3 →(+3e) Al
Aluminium ion → Aluminium Metal
At Anode:-
O-2 →(-2e) O
O + O → O2
During this process, all the carbon anodes are gradually consumed. This is due to the reason that the oxygen gas liberated at anode reacts with carbon anodes to form CO2 gas. So, it is necessary to replace carbon anodes from time to time.
C(s) + O2(g) → CO2(g)
Carbon + Oxygen → Carbon dioxide
3. Refining of Aluminium
The aluminium metal produced by the electrolysis of molten aluminium oxide contains some impurities. These impurities can be removed by the electrolytic refining method.
Uses of Aluminium
- Aluminium is used for making cooking utensils, aluminium foils, television aerials, ships, aeroplanes and space rockets.
- It is used in aluminotherapy for joining the broken pieces of heavy iron objects like girders, rail or heavy machinery.
- It is used for making alloys such as duralumin and magnelium.
- It is used as a reducing agent in metallurgy.
- It is used in electric transmission lines.
Test your understanding and answer these questions:
- Name two ores of aluminium metal?
- Explain the process of extraction of aluminium metal from bauxite ore.
- What are uses of aluminium metal?
