You have already learnt that there are certain chemical reactions which produce electricity. But, it is interesting to note that when electric current is passed through certain liquids a chemical reaction takes place. This is called chemical effect of electric current. For example, when an electric current is passed through acidified water, it splits up to form hydrogen and oxygen gas. Let’s understand the chemical effect of current more deeply by discussing various terms and laws related with it.
Electrolytes
The substances which allow electricity to pass through them when dissolved in water or in molten state and dissociate into ions on passing the current through them are called electrolytes. For example, solution of sodium chloride in water and solution of copper sulphate in water are electrolytes.
Electrolysis
The process of dissociation of an electrolyte into ions on passing the electric current through it is called electrolysis. For example, when an electric current in passed through aqueous solution of sodium chloride it splits up into positively charged sodium ions and negatively charged chloride ions.
NaCl ![]() Na+ + Cl–
Na+ + Cl–
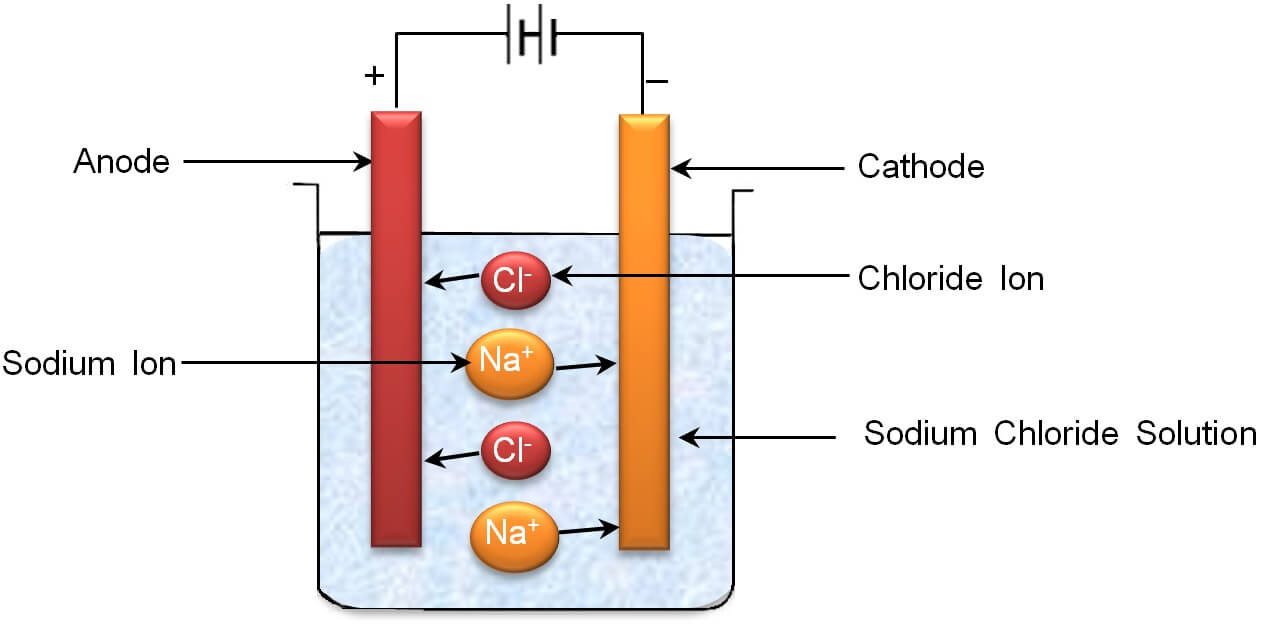
Remember that the vessel in which the process of electrolysis is carried is called voltameter.
Test Your Understanding and Answer These Questions:
- What is chemical effect of electric current?
- What do you meant by electrolytes? Give examples.
- What is electrolysis process? Give an example.
- What do you understand by voltameter?
