Take three test tubes A, B and C and place one clean iron nail in each of them. In the test tube A pour some water and close its mouth with the help of a cork. In the test tube B pour boiled water which does not contain dissolved air. Also pour some oil in test tube B to form a layer over boiled water. The layer of oil will prevent the entry of air in water. In the test tube C put some anhydrous calcium chloride and cork it. Anhydrous calcium chloride is drying agent. So, it is added into test tube C to absorb all the moisture present in the air of test tube. Keep these test tubes undisturbed for some days.
After some days, you will observe that the nail in test tube B does not rust because the nail was exposed only to water and no air was present in it. In the same way the nail present in test tube C does not rust because in this tube air is dry and water is not present. But the nail present in test tube A rusts because in test tube A the nail was exposed to both air and water.
This shows that for rusting of iron both air and water are necessary.
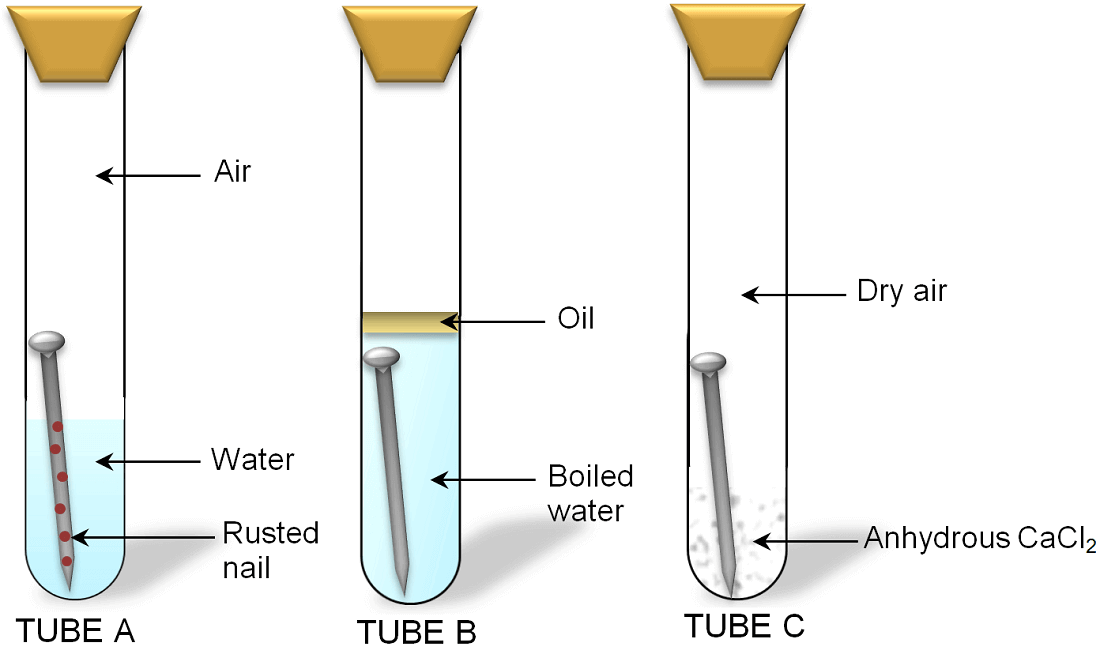
Investigation the conditions under which iron nail rusts
Test your understanding and answer these questions:
- Give an experiment to prove that both air and water are necessary for rusting of iron.
