The elements in which the last electron enters the f-orbitals of their atoms are called f-block elements. These consist of two series of elements placed at the bottom of the periodic table.
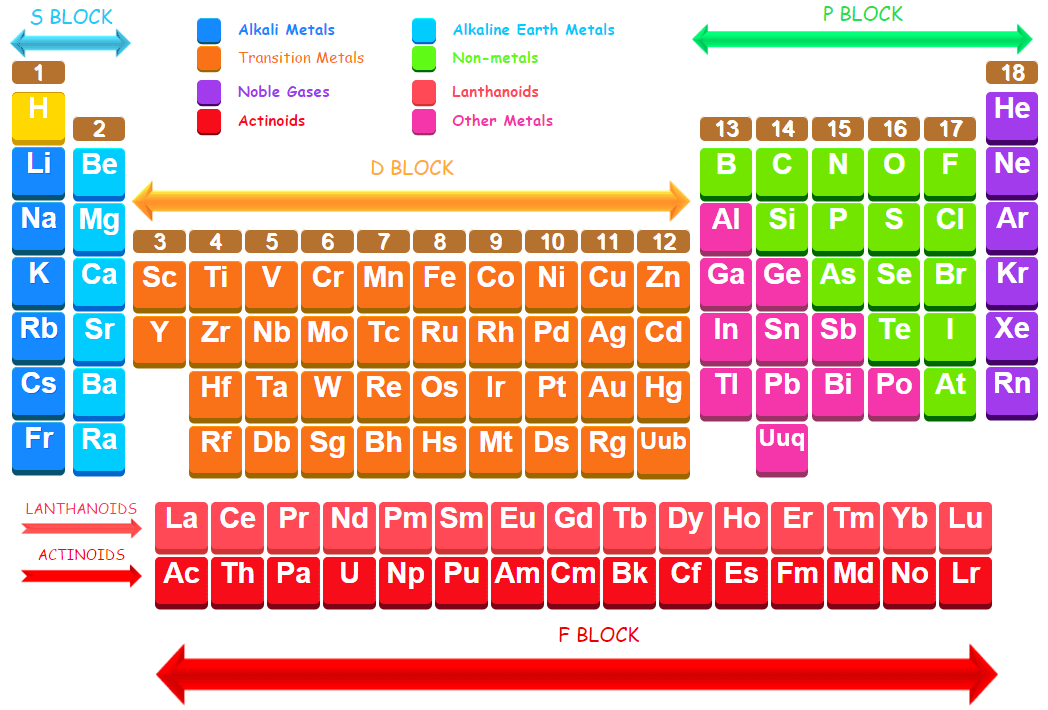
Lanthanoids or Lanthanides or rare earth metals: The first series follows lanthanum, La (Z= 57) and the elements present in this series from cerium to lutetium (58Ce – 71Lu) are called lanthaniods or lanthanides. These are also called rare earth elements.
Actinoids or Actinides: The second series follows actinium, Ac (Z = 89) and the elements present in this series from thorium to lawrencium (90Th – 103Lr) are called actionoids or actinides. These are of radioactive elements.
The general electronic configuration of f-block elements may be written as: (n-2) f1-14 (n-1) d0-2 ns2 The elements included in these two series are called inner transition elements, because they form transition series within the transition elements of d-block.
General characteristics of f-block elements.
- They are heavy metals.
- They generally have high melting and boiling points.
- They exhibit variable oxidation states.
- They form coloured ions.
- They have the tendency to form complex compounds.
- Actinoids are radioactive in nature. The elements after uranium are called transuranium elements.
Test your understanding and answer these questions:
- What are lanthanoids or lanthanides?
- What are actinoids or actinides?
- Why are f block elements called inner transition elements?
- What is the general electronic configuration of f block elements?
