The modern periodic table was constructed by the Russian chemist Dmitri Mendeleyev in 1869. The modern periodic table consists of horizontal rows called periodsand vertical columns called groups. These are discussed below:
Groups in Modern Periodic Table
A group may be defined as vertical column in the periodic table. In terms of electronic structure of the atom, a group constitutes a series of elements whose atoms have the same outermost electronic configurations. There are 18 groups in the long form of the periodic table. According to the new recommendations ofinternational Union of Pure and Applied Chemistry (IUPAC), the groups are numbered from 1 to 18.
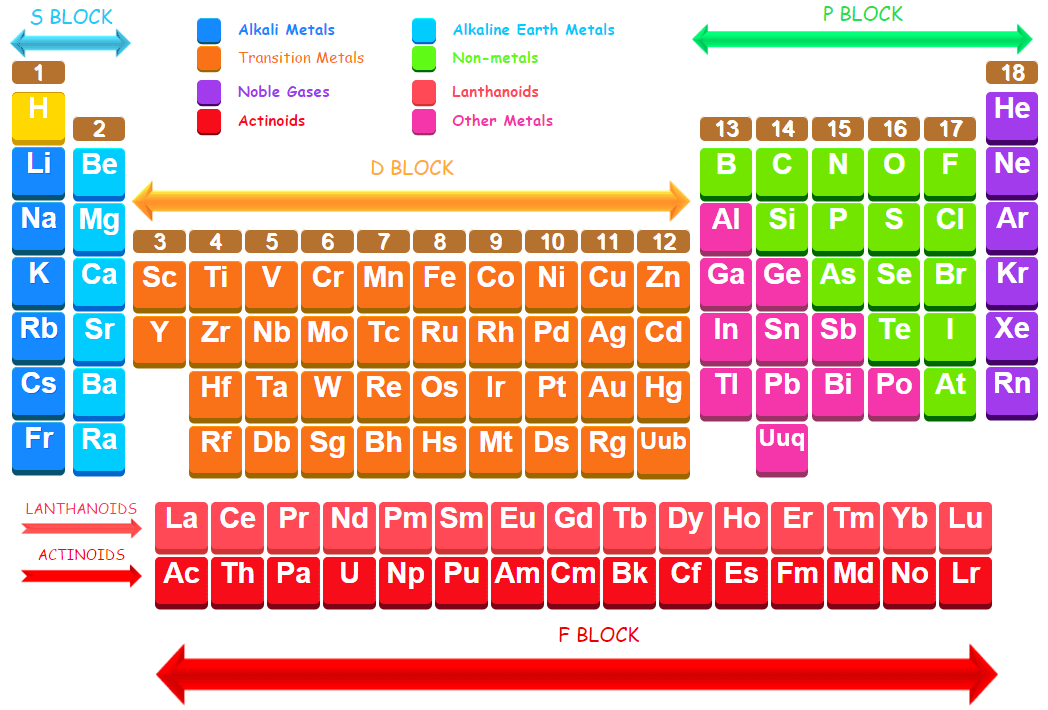
Each group contains elements that tend to react chemically in similar ways, because they all have atoms in which the arrangement of electrons around the nucleus is similar. Some groups in the periodic table are given special names e.g.
Group 1 the alkali metals
Group 2 the alkaline earth metals
Group 15 the pnicogens
Group 16 the chalcogens
Group 17 the halogens
Group 18 the noble gases
Representative elements in modern periodic table
The first two groups on the extreme left and last six groups on the extreme right involve the filling of s- and p- orbitals, respectively, These groups represent the main groups of the periodic table and are numbered as 1, 2, 13, 14, 15, 16, 17 and 18. The elements present in these groups are known as normal elements or representative elements.
Transition series elements in modern periodic table
The ten groups lie in between first two and last six groups i.e. between group 2 and group 13. These are numbered from 3 to 12. The elements present in these groups are called transition elements. The name is derived from the fact that they represent transition (change) in character from reactive metals (elements of groups 1 and 2) on one side to the non-metals (elements of group 13 to 18) on the other side.
Inner transition series elements in modern periodic table
There are two more rows at the bottom of the periodic table. These rows consist of fourteen elements after lanthanum (Z = 57) and fourteen elements which follow actinium (Z = 89). These are placed separately in the periodic table to save space and avoid undue sidewise expansion of the periodic table. The elements in the first row, starting from cerium are called lanthanoids (or lanthanides) and the elements present in the second row starting from thorium are called actinoids (or actinides). These lanthanoids and actinoids together are called inner transition elements or rare earth metals and these are built up by filling of f-orbitals.
Test your understanding and answer these questions:
- What are alkali metals?
- What are alkaline earth metals?
- What are representative elements?
- What is transition series of elements?
- What is inner transition series of elements?
- How many groups are there in modern periodic table?
- What is definition of group in modern periodic table?
