Pure Iron is a grey-white metal. It appears to be brown due to rusting. Iron is magnetic, malleable and ductile. It is a good conductor of heat and electricity.
Occurrence of Iron
Iron is the second most abundant metal in earth’s crust. It is not found in a free state in the earth because it is quite reactive. So, it is found in the form of its oxides, carbonates and sulphides. The main ores of Iron are:
1. Haematite Fe2O3
2. Magnetite Fe3O4
3. Siderite FeCO3
4. Iron Pyrite FeS2
Extraction of Iron from Haematite
Iron is extracted from its chief ore haematite by reducing haematite in a big furnace called a blast furnace. The steps involved in the extraction of iron are:
1. Concentration of Haematite
Haematite is concentrated by the process of hydraulic washing. In this method, haematite is crushed to small pieces and then washed in a stream of water to remove impurities (sand and clay) from it.
2. Calcination
The washed haematite is then heated strongly in the absence of air to expel water from it.
3. Reduction
The washed and dried haematite is now mixed with coke and limestone. The mixture of haematite, coke and limestone is called charge. The charge is then fed into the blast furnace from the top. A blast of the hot air which is at about 800°C temperature is forced in the furnace through tubes present at the bottom. As the hot air rises upward it comes into contact of coke and the following two reactions take place in a blast furnace.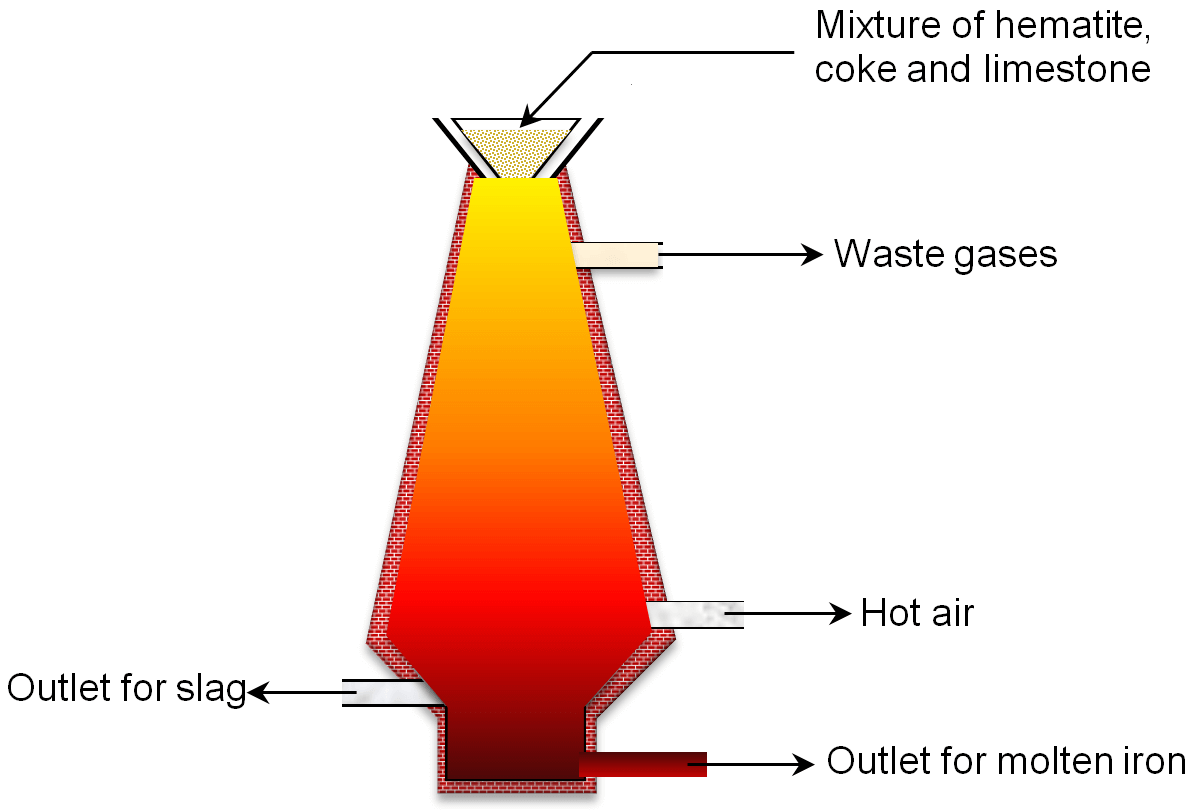
1) Formation of Carbon Monoxide
As the hot air comes in contact with coke carbon dioxide is formed. In this reaction, a large amount of heat is produced which raises the temperature of the furnace further to 1500°C. This carbon dioxide rises upward and reacts with more coke to form carbon monoxide. Carbon monoxide is the main reducing agent in the extraction of iron by blast furnace.
C + O2 → CO2 + Heat
Coke + Oxygen → Carbon dioxide + Heat
CO2 + C → 2CO
Carbon dioxide + Coke → Carbon monoxide
2) Reduction of Haematite to Iron
The carbon monoxide reduces haematite into iron metal in the upper part of the furnace.
Fe2O3 + CO → 2Fe + 3CO2
Haematite + Carbon monoxide → Iron Metal + Carbon dioxide
The iron so produced is in liquid form and collects at the bottom of blast furnace.
Function of Limestone in Extraction of Iron
The haematite used in the blast furnace always contains sand as impurties. The sand has a high melting point. So, it does not change to liquid in the furnace. Thus, in order to remove sand from iron limestone is added in the blast furnace.
In the blast furnace limestone decomposes to form CaO and CO2. CaO produced from the lime stone acts as flux. It reacts with sand to form molten calcium silicate referred to as slag.
CaCO3 + CaO → CO2
Limestone + Calcium oxide → Calcium oxide
CaO + SiO2 → CaSiO3
Calcium oxide (flux) + Sand → Calcium silicate (slag)
The molten calcium silicate slag is lighter than iron. So, it floats over molten iron and removed. The iron obtained from the blast furnace is called cast iron or pig iron.
Test your understanding and answer these questions:
- Name ores of iron metal?
- Explain the process of extraction of iron metal from haematite ore.
- What is pig iron?
- What is flux and slag?
