Definition of Isotopes: Isotopes of an element are the atoms of the element which have same atomic number but different mass number.
Examples of Isotopes
Isotopes of Hydrogen
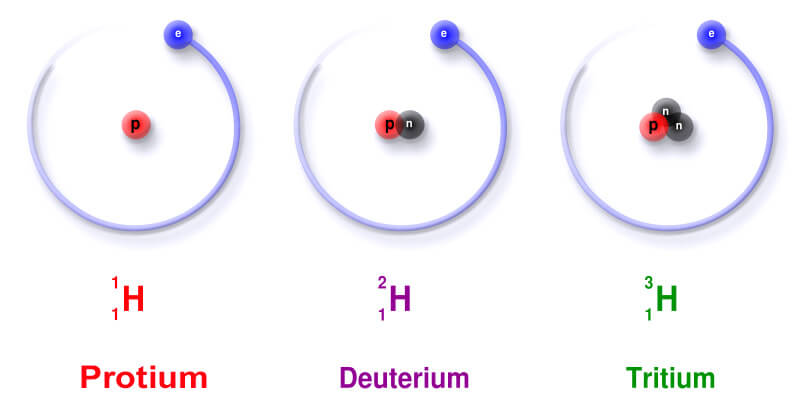
Hydrogen element also exists in the form of three isotopes: protium (1H1), deuterium (1H2) and tritium (1H3). All the three isotopes of hydrogen element contain 1 proton so they have same atomic number. But, the number of neutrons present in each of them is different so they have different mass numbers.
In protium (1H1) no neutron is present, in deuterium (1H2) the number of neutrons present is one and in tritium (1H3) the number of neutrons present is two.
First isotope of hydrogen i.e. protium is present in normal water (H2O), second isotope of hydrogen i.e. deuterium is present in heavy water (D2O) whereas third isotope of hydrogen i.e. tritium is radioactive isotope and found in trace amount in nature.
Isotopes of Uranium:
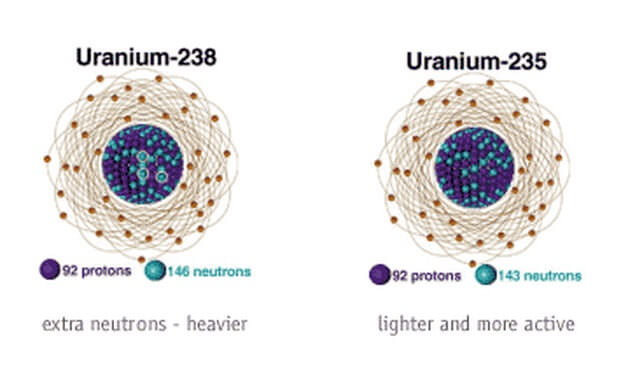
92U235 and 92U238 are the two isotopes of element uranium, because both uranium atoms have same atomic number of 92 but different mass numbers of 235 and 238 respectively. The first isotope of uranium i.e. 92U235 is a normal isostope whereas second isotope of uranium i.e. 92U238 is a radioactive isotope.
It should be noted that as the atomic number of isotopes of an element are same, the number of protons present in them is also same. But as their mass numbers are different, the number of neutrons present in them is different. For example, in 92U235 there are present 92 protons, 92 electrons and 143 neutrons. But in 92U238 there are present 92 protons, 92 electrons and 146 neutrons. So, both the isotopes of uranium contains same number of protons i.e. both isotopes have 92 protons. But the number of neutrons present in both isotopes is different i.e. the number of neutrons present in both isotopes is 143 and 146 respectively.
Test Your Understanding and Answer These Questions:
- What is isotopes?
- What are 3 isotopes of hydrogen?
- What are 2 isotopes of uranium?
