The radioactive rays are of three types:
- Alpha rays
- Beta rays
- Gamma rays
Nature and Characteristics of Alpha Rays
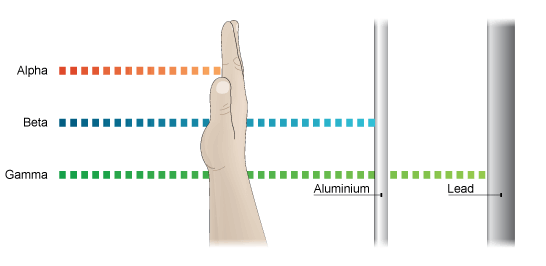
The alpha rays consist of very small positively charged particles called alpha particles. The alpha particles may be considered as helium nuclei because they have a mass of 4 a.m.u. and carries 2 units of positive charge (+2). The alpha particles are represented by symbol 2He4. The average velocity of an alpha particle is about 1/10th of that of light. Alpha rays have very poor penetrating power. These cannot pass even pass through a thin sheet of paper.
Nature and Characteristics of Beta Rays
The beta rays consist of very small negatively charged particles called beta particles. Beta particles have no mass but they carry 1 unit negative charge (-1). Beta particles may be represented by the symbol -1e0. The average velocity of a beta particle is equal to that of light. Beta particles have penetrating power more than alpha rays. These can easily pass through a thin sheet of paper but are stopped by thin sheet of aluminium metal.
Nature and Characteristics of Gamma Rays
Gamma rays consist of neutral particles which have no mass and charge. These are represented by the symbol 0γ0. The average velocity of gamma rays is also equal to that of light. Gamma rays are the radiations of highest energy. Gamma rays can easily pass through aluminium sheets but are stopped by a thick block of lead metal.
Test Your Understanding and Answer These Questions:\
- What are different types of radioactive radiations?
- What is symbol of alpha particles?
- What is symbol of beta particles?
- What is symbol of gamma rays?
