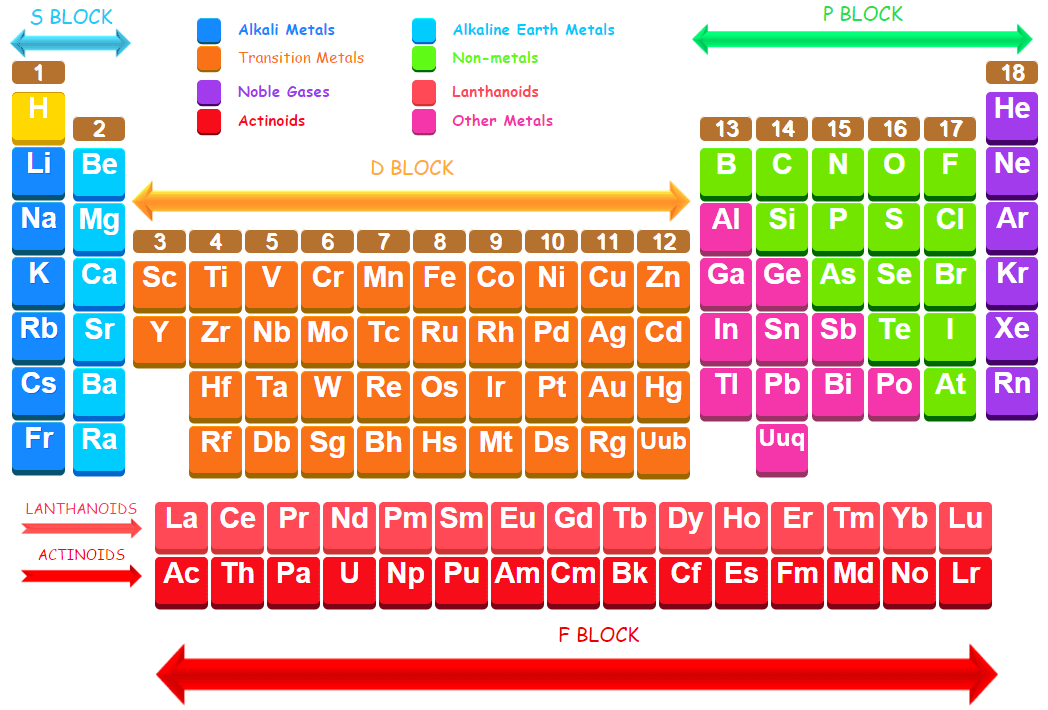
All the existing elements in the modern periodic table is divided into four main segments or blocks. A block consists of groups of elements having similar electronic configurations and properties. These are called s block, p block, d block and f-block.
S block elements on modern periodic table
The elements in which the last electron enters the s-orbital of their outermost energy level are called s-block elements. It consists of elements of group 1 of modern periodic table and group 2 of modern periodic table having the ground state electronic configurations of outermost shell as ns1 and ns2 respectively (where n stands for outermost energy shell). The elements of first group on modern periodic table corresponding to ns1 configuration are called alkali metals while the elements of group 2 on modern periodic table corresponding to ns2 configuration are called alkaline earth metals. Thus the general electronic configuration of s-block elements may be expressed as : ns1 – 2
General characteristics of s-block elements
- They are soft metals having low melting and boiling points.
- They have low ionization enthalpies.
- They are very reactive and readily form univalent ions (alkali metals) or bivalent ions (alkaline earth metals) by losing one or two valence electrons respectively.
- Most of them impart characteristic colours to the flame.
- They mostly form ionic compounds except lithium and beryllium.
Test your understanding and answer these questions:
- What are alkali metals?
- What are alkaline earth metals?
- What is the general electronic configuration of s block elements?
