Generally chemical reactions take place by transfer or sharing of valence electrons. These reactions do not cause any change in the nucleus of atom. But, there are certain reactions in which nucleus of atoms undergo a change. Such reactions in which nuclei of atoms undergo a change are called nuclear reactions. For example, radioactivity, nuclear fission and nuclear fusion are nuclear reactions. During nuclear reactions, a large amount of energy is produced in the form of heat and light. The energy produced during a nuclear reaction is called nuclear energy.
Now, we shall discuss the structure of an atom in detail in order to understand nuclear reactions more clearly.
Structure of Atom
An atom consists of three fundamental particles: electrons, protons and neutrons. The electrons have negative charge; protons have positive charge while the neutrons have no charge. Structurally, an atom is divided into two parts: nucleus and extra nuclear portion. The nucleus is a tiny, heavy sphere which is present at the centre of an atom. The nucleus of an atom consists of protons and neutrons. Nucleus is positively charged due to the presence of protons in it. The diameter of nucleus ranges from 10–5 to 10–4 m.
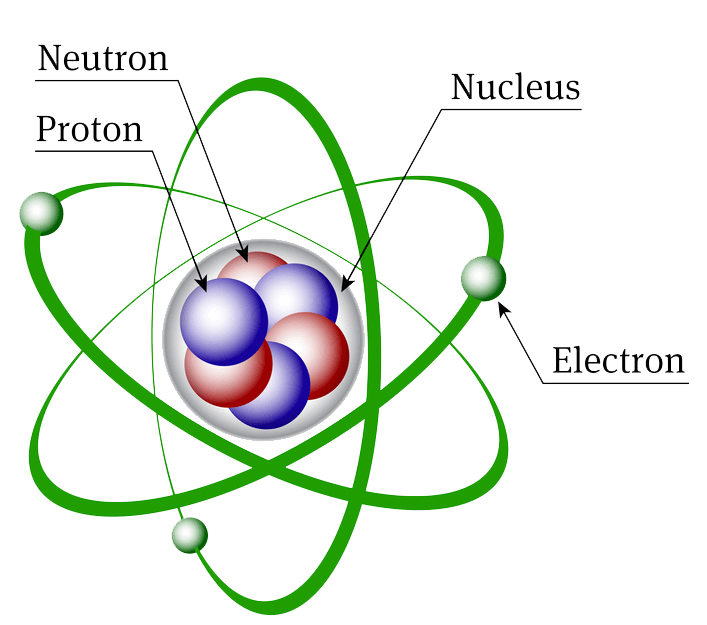
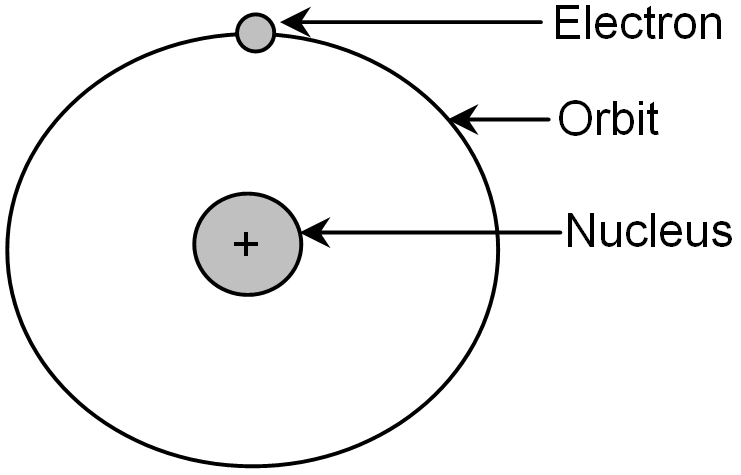
The extra nuclear portion consists of negatively charged electrons which revolves around the positively charged nucleus in circular paths called orbits. In an atom, the number of electrons is always equal to that of protons. So, atoms are always electrically neutral.
Model of an atom
Test Your Understanding and Answer These Questions:
- What is definition of nuclear reaction?
- What is definition of nuclear energy?
- What is the structure of an atom?
- Describe model of an atom.
