Sulphur is a non metal having atomic number 16. So, it electronic configuration is 2.8,6. It is represented by symbol S. it is placed in group 16 of the periodic table.
Occurrence of Sulphur
Sulphur occurs both in free state and combined state in nature. In free state sulphur is found in large amount in the form of sulphur beds under the earth. In combined state sulphur occurs in the form of sulphides of many metals such as iron, copper, silver, zinc, lead, and mercury. For example,
1. Iron pyrite (FeS2)
2. Copper pyrite (CuFeS2)
3. Silver glance (Ag2S)
4. Zinc blende (ZnS)
5. Galena (PbS)
6. Cinnabar (HgS)
Structure of Sulphur Molecule(S8)
One molecule of sulphur contains 8 atoms of sulphur. These 8 atoms of sulphur combine with each other to give a puckered ring structure to sulphur molecule.
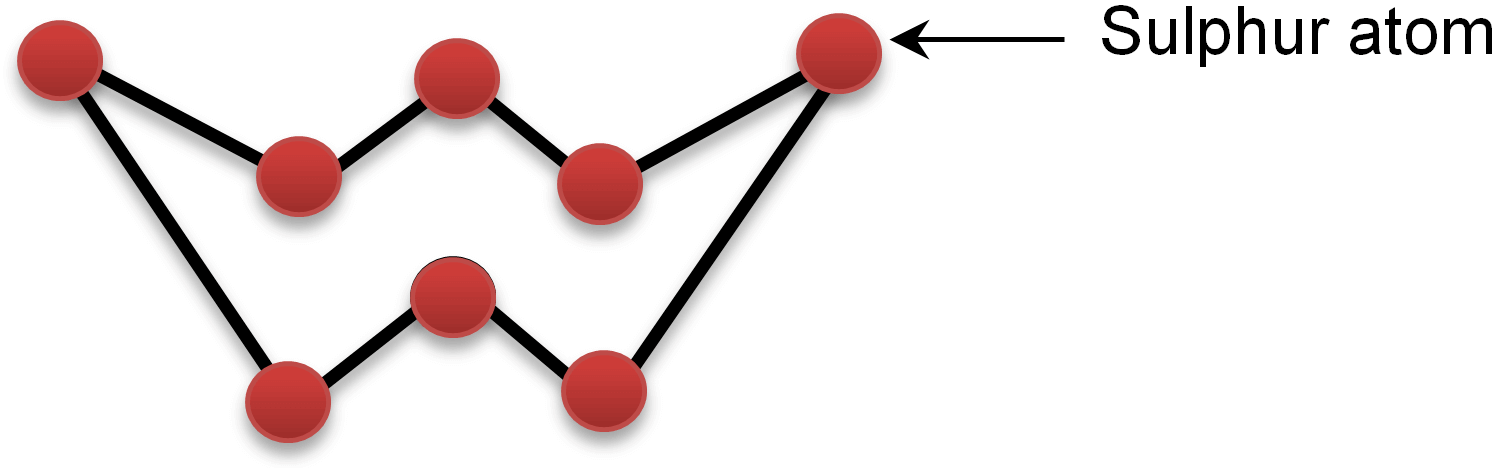
Puckered ring structure of sulphur molecule
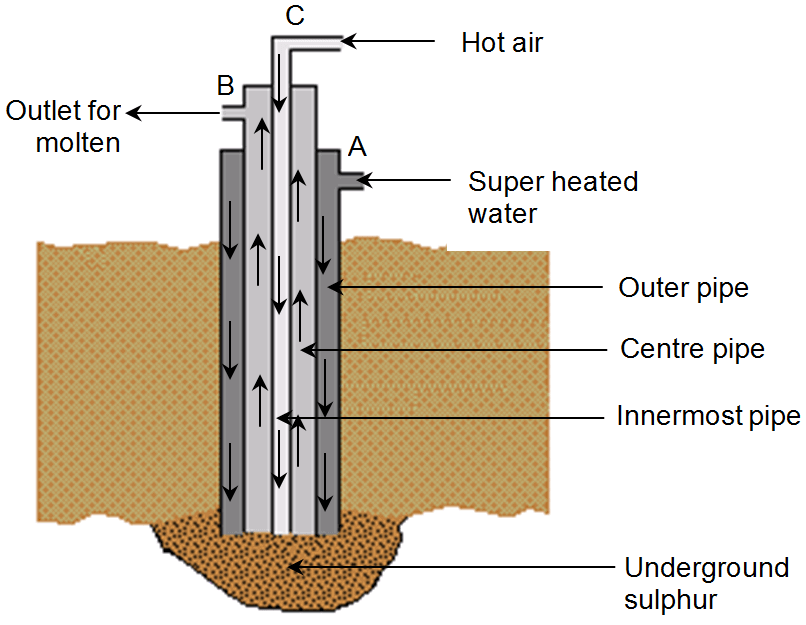
Extraction of Sulphur (Frasch Process)
Sulphur occurs in free state under the surface of earth. It is extracted by Frasch process, which is based on the fact that sulphur has low melting point of 115°C. In this process, a hole is bored in the earth up to sulphur bed. Three concentric pipes A, B, C of different diameter are placed in the hole. Superheated water is forced down through the outer pipe A in to the sulphur bed. The super heated water melts the sulphur. Now, through innermost pipe C hot and compressed air is forced down. The force of hot compressed air brings the molten sulphur at the surface of earth through middle pipe B.
Physical Properties of Sulphur
- It is a yellow crystalline solid, having no taste and odour.
- It is a poor conductor of electricity and heat, thus it acts as an insulator.
- It has a melting point of 115°C.
- It is insoluble in water but freely soluble in carbon disulphide solution.
- The vapours of sulphur are poisonous for bacteria and fungi but not for human beings.
- It exhibits allotropy. The important allotropes of sulphur are rhombic sulphur and monoclinic sulphur.
Chemical Properties of Sulphur
1. Burning of Sulphur
On strong heating sulphur burns in air with a blue flame to form sulphur dioxide.
S + O2 → SO2
(Sulphur + Oxygen → Sulphur dioxide)
2. Reaction with Metals
Sulphur reacts with many metals to form metal sulphides. These are exothermic reactions in which heat is evolved. For example,
Fe + S → FeS + Heat
(Iron + Sulphur → Iron sulphide + Heat)
Zn + S → ZnS + Heat
(Zinc + Sulphur → Zinc sulphide + Heat)
Cu + S → CuS + Heat
(Copper + Sulphur → Copper sulphide + Heat)
3. Reaction of Sulphur with Non-metals
Sulphur reacts with non-metals to form covalent sulphides. For example,
1) Reaction with Hydrogen:- Sulphur reacts with hydrogen to form hydrogen sulphide.
S + H2 → H2S
(Sulphur + Hydrogen → Hydrogen sulphide)
2) Reaction with Carbon:- Upon heating sulphur reacts with carbon to form carbon disulphide
C + 2S → CS2
(Carbon + Sulphur → Carbon disulphide)
4. Reaction with Acids
Sulphur is oxidised to sulphur dioxide by hot concentrated sulphuric acid.
Sulphur is oxidised to sulphuric acid by concentrated nitric acid.
S + 2H2SO4 → 3SO2 + 2H2O
(Sulphur + Sulphuric acid → Sulphur dioxide + Water)
S + 6HNO3 → H2SO4 + 6NO2 + 2H2O
(Sulphur + Nitric acid → Sulphuric acid + Nitrogen dioxide + Water)
Effect of Heat on Sulphur
Following are the effect of heat on a sulphur molecule:
- When sulphur is heated to its melting point (115°C) it melts and forms a pale yellow liquid which flows freely like water.
- On further heating to about 160°C, sulphur becomes viscous and dark in colour.
- On further heating to about 440°C, the molten sulphur becomes light in colour and less viscous.
- At 444°C molten sulphur begins to boil producing yellow brown vapours.
Uses of Sulphur
- The major use of sulphur is in the manufacturing of sulphuric acid.
- It is used in the manufacture of carbon disulphide, gun powder, matches and in fireworks.
- It is used in vulcanization of natural ruber.
- It is used as an antiseptic in making skin ointments.
- It is used as a disinfectant, fungicide and insecticide for killing bacteria, fungi and insects.
- It is also used in the manufacture of sodium thiosulphate which is used in photography.
Test your understanding and answer these questions:
- Name ores of sulphur?
- Explain Frasch process of manufacture of sulphur.
- What are uses of sulphur?
- What are properties of sulphur?
- Explain structure of sulphur molecule.
