Voltaic cell is the simplest and earliest device which is capable of converting chemical energy into electrical energy. It was invented by Alexandro de Volta in 1800.
Construction and Working of Voltaic Cell
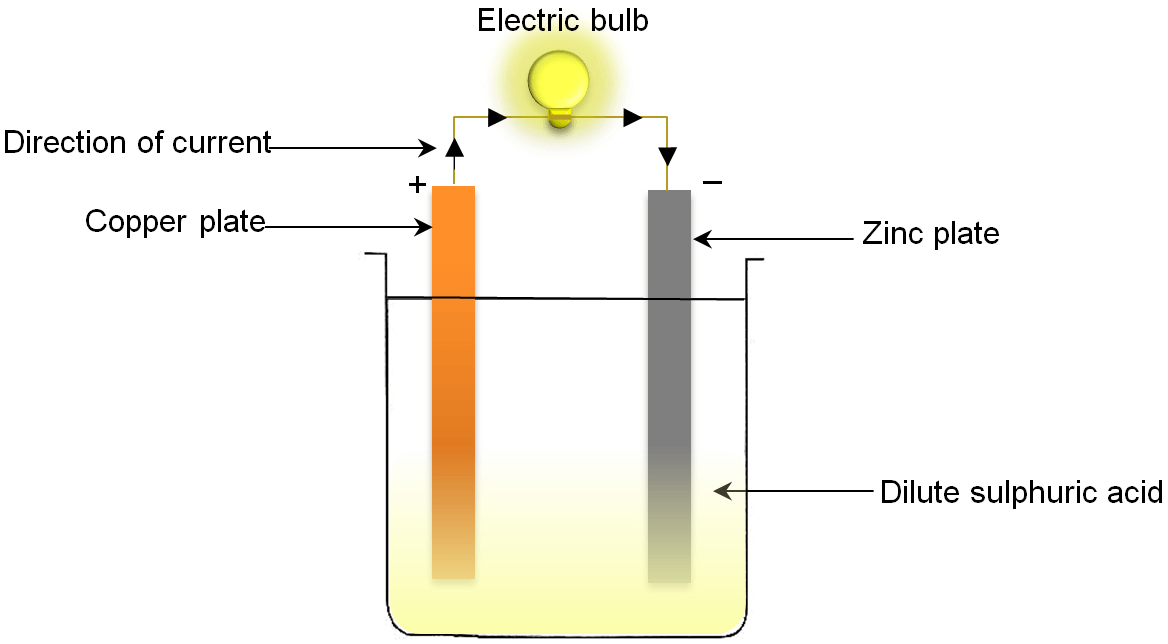
A simple voltaic cell can be constructed in laboratory. For this, take two rods one of copper and other of zinc and dip them partially in solution of dilute sulphuric acid contained in a glass jar. Connect these two rods to a small bulb with the help of copper wire as shown in figure. When the rods are connected to bulb, the bulb lights up because the circuit is completed and a chemical reaction takes place in the glass jar. Due to this chemical reaction the zinc rod acts as negative electrode while the copper rod acts as positive electrode. Thus, a potential difference is set up between the copper rod and zinc rod of the cell. This potential difference causes the electrons to flow from the negatively charged zinc rod to the positively charged copper rod through copper wire. But by convention the direction of flow of current in a cell is opposite to that of flow of electrons. So, the current is said to flow from positive copper electrode to negative zinc electrode.
Test Your Understanding and Answer These Questions:
- What is voltaic cell?
- How invented voltaic cell?
- How does voltaic cell work?
